cell-membrane-mimicking lipid coated silver-nanoparticles for deducing membrane-protein structure
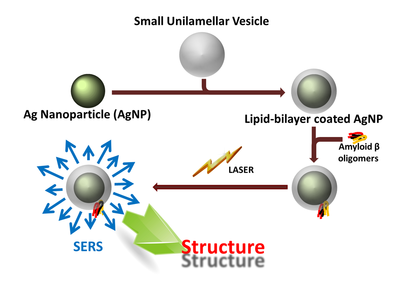
A key feature of many protein-aggregation linked human diseases, such as Alzheimer’s (AD), Parkinson’s and Type II diabetes, is the existence of a small soluble aggregate species which display strong affinity to cell membranes. The structure of this species and the nature of its interaction with membranes have therefore attracted intense scrutiny. In the case of Alzheimer’s amyloid beta, some solution state structural parameters of the early oligomeric species have been obtained recently. This is the earliest aggregate to display a high affinity for lipid bilayers. These data suggest an overall structural topology similar to the less toxic mature fibrils, with some significant differences in specific segments. Very little is known about the conformation of these early oligomers in the lipid bilayer. The low concentrations of this species, and the simultaneous presence of other low-affinity species (such as the monomers) in the solution, make this problem rather complex. Conventional techniques to obtain structure or conformation, such as NMR, circular dichroism or vibrational spectroscopy, are not easily applied to a peptide present at low μM concentrations, especially when only a fraction of it is attached to the membrane. However, since Aβ is an extracellular peptide, the conformation of this species in the membrane-bound state may hold the key to understanding disease initiation. We demonstrate a Surface Enhanced Raman Spectroscopy (SERS) based technique which can determine the conformation of membrane-bound proteins, even at low μM concentrations, and even in the presence of a substantial membrane-free fraction. Conventional SERS requires immobilization of molecules, and is also not applicable to membrane proteins. We circumvent these limitations by using spontaneous binding of proteins to lipid bilayer-encased Ag nanoparticles.
Here we demonstrate a strategy to preferentially enhance the Raman signal from the membrane attached fraction, using SERS from silver nanoparticles (AgNPs). We follow a technique to encapsulate AgNPs with a lipid bilayer. We incubate the peptide of interest with these particles, and allow them to spontaneously attach to the lipid membrane. The plasmon enhancement is effectively present only for a few nanometers from the nanoparticle surface. Thus only the species which are bound to the lipid bilayer coating would experience the SERS effect and the free population in solution would not. This also helps avoid the artefacts associated with covalent attachment of peptides with nanoparticles. We first demonstrate our method by investigating the water channel protein gramicidin A. This protein is known to have very high affinity to the membrane, and the crystal structure and the Raman spectra of this peptide are well studied. Our SERS experiment shows clear peaks at 1650 cm-1 and at 1573 cm-1, which are in agreement with earlier bulk Raman studies performed (with a high concentration of gramicidin A) by Krimm's group. This demonstrates the potential of our technique to deduce the structures of membrane-bound proteins.
We then probe membrane-attached oligomers of Amyloid-β40 (Aβ40), whose conformation is keenly sought in the context of Alzheimer’s disease. Our results suggest a near-orthogonal orientation of the backbone H-bonds compared to the mature Aβ fibrils. Interestingly, this would allow a “porin” like β–barrel structure, providing a structural basis for the membrane permeabilization hypothesis about the mechanism of Aβ oligomer toxicity. For details please click the following link.
Here we demonstrate a strategy to preferentially enhance the Raman signal from the membrane attached fraction, using SERS from silver nanoparticles (AgNPs). We follow a technique to encapsulate AgNPs with a lipid bilayer. We incubate the peptide of interest with these particles, and allow them to spontaneously attach to the lipid membrane. The plasmon enhancement is effectively present only for a few nanometers from the nanoparticle surface. Thus only the species which are bound to the lipid bilayer coating would experience the SERS effect and the free population in solution would not. This also helps avoid the artefacts associated with covalent attachment of peptides with nanoparticles. We first demonstrate our method by investigating the water channel protein gramicidin A. This protein is known to have very high affinity to the membrane, and the crystal structure and the Raman spectra of this peptide are well studied. Our SERS experiment shows clear peaks at 1650 cm-1 and at 1573 cm-1, which are in agreement with earlier bulk Raman studies performed (with a high concentration of gramicidin A) by Krimm's group. This demonstrates the potential of our technique to deduce the structures of membrane-bound proteins.
We then probe membrane-attached oligomers of Amyloid-β40 (Aβ40), whose conformation is keenly sought in the context of Alzheimer’s disease. Our results suggest a near-orthogonal orientation of the backbone H-bonds compared to the mature Aβ fibrils. Interestingly, this would allow a “porin” like β–barrel structure, providing a structural basis for the membrane permeabilization hypothesis about the mechanism of Aβ oligomer toxicity. For details please click the following link.
