Amyloid β (Aβ) Monomers structure and monomer to oligomer transition:
A variety of neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s disease, have been shown to correlate with aggregation of specific proteins and peptides. In case of Alzheimer’s disease that role is played by an extracellular peptide Amyloid β (Aβ). As aggregation occurs, Aβ changes it structure and thereby also its function. Understanding of these structural transitions, in each possible step of aggregation, is a necessary requirement for understanding the toxic functional change associated with it. However, in physiological conditions determination of these evolving structures are still open challenge(s), owing to low solubility, heterogeneity and transient nature of some particular species in the aggregation pathway. As different species evolves during aggregation, isolation and stabilization of all possible individual species is necessary to deduce any structural insight.
Surface Enhanced Raman Spectroscopy (SERS) to deduce monomer conformation: As a first step we have tried to determine the conformation of Amyloid-β40 monomers in solution at physiological pH and temperature by the help of SERS. In this experiment we have attached monomers of Aβ1-40 (having an extra cysteine at the C-terminus, Aβ40-Cys) directly on to silver nanoparticle surfaces and have seen the monomer structure being predominantly α-helical.
A variety of neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s disease, have been shown to correlate with aggregation of specific proteins and peptides. In case of Alzheimer’s disease that role is played by an extracellular peptide Amyloid β (Aβ). As aggregation occurs, Aβ changes it structure and thereby also its function. Understanding of these structural transitions, in each possible step of aggregation, is a necessary requirement for understanding the toxic functional change associated with it. However, in physiological conditions determination of these evolving structures are still open challenge(s), owing to low solubility, heterogeneity and transient nature of some particular species in the aggregation pathway. As different species evolves during aggregation, isolation and stabilization of all possible individual species is necessary to deduce any structural insight.
Surface Enhanced Raman Spectroscopy (SERS) to deduce monomer conformation: As a first step we have tried to determine the conformation of Amyloid-β40 monomers in solution at physiological pH and temperature by the help of SERS. In this experiment we have attached monomers of Aβ1-40 (having an extra cysteine at the C-terminus, Aβ40-Cys) directly on to silver nanoparticle surfaces and have seen the monomer structure being predominantly α-helical.
Aggregation or Conformation change: What happens first?
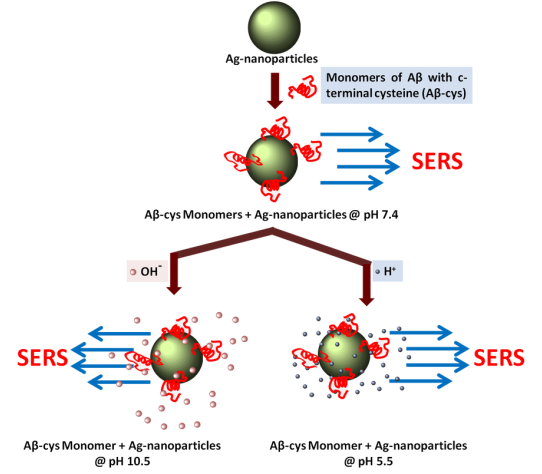
As a next step we deduce the structure of small oligomers to be predominantly β-sheet by SERS. Since the structural transition was observed upon formation of oligomers from monomers, the age old question arises again that the Aggregation or Conformation change: which precedes what? To answer this challenging question, the requirement is to decouple the aggregation from the conformation change. Otherwise conformation changes in the monomeric state in a aggregation favoring condition would be extremely transient before it quickly forms oligomers.
Grafting of Aβ40–Cys on nanoparticle surface in a non-interactive distance from each other gives us just that opportunity to study the conformation of monomeric Amyloid beta, decoupled from the aggregation by Surface Enhanced Raman Spectroscopy (SERS).We then put these particles at pH 5.5 (favorable condition for Aβ aggregation) and at pH 10.5 (favorable condition for high Aβ solubility).
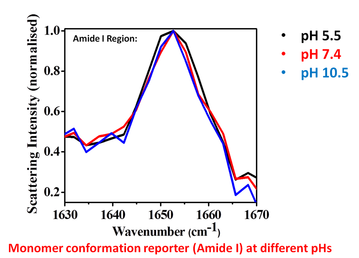
With change in pH, both in aggregation favorable and unfavorable conditions the Amide I peak (in the SERS spectra) remains unchanged for the monomer of Aβ40–Cys. This indicates structural ensemble remains the same and it is the aggregation which happens first leading to conformational changes in the peptide in the oligomeric state only.
As a next step we deduce the structure of small oligomers to be predominantly β-sheet by SERS. Since the structural transition was observed upon formation of oligomers from monomers, the age old question arises again that the Aggregation or Conformation change: which precedes what? To answer this challenging question, the requirement is to decouple the aggregation from the conformation change. Otherwise conformation changes in the monomeric state in a aggregation favoring condition would be extremely transient before it quickly forms oligomers.
Grafting of Aβ40–Cys on nanoparticle surface in a non-interactive distance from each other gives us just that opportunity to study the conformation of monomeric Amyloid beta, decoupled from the aggregation by Surface Enhanced Raman Spectroscopy (SERS).We then put these particles at pH 5.5 (favorable condition for Aβ aggregation) and at pH 10.5 (favorable condition for high Aβ solubility).
With change in pH, both in aggregation favorable and unfavorable conditions the Amide I peak (in the SERS spectra) remains unchanged for the monomer of Aβ40–Cys. This indicates structural ensemble remains the same and it is the aggregation which happens first leading to conformational changes in the peptide in the oligomeric state only.
