Live Cell Interaction
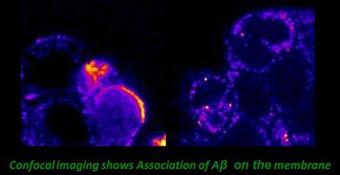
Aggregation of Aβ takes place in the extra-cellular environment, in presence of or on the membrane. The aggregation behaviour of the peptide can in principle be very different in such an environment. We have investigated amyloid protein aggregation on the cell membrane and in the cytoplasm of living PC12 cells. Simultaneous confocal imaging, Fluorescence Correlation Spectroscopy and Lifetime imaging were employed to address these questions. Our lifetime data suggests that the peptide does incorporate into the membranes, while our FCS data shows that they do form aggregates on the membrane. We find that Aβ can aggregate on living cell membranes at concentrations much lower than that required for its aggregation in the aqueous solution. This answers the puzzle that while it takes multiple µM concentration of Aβ for the aggregation to take place in vitro, the in vivo concentrations even in a diseased brain is less than 1/10th of that value. [Nag et al., Biophys. J., 2010; Collaborator: J. Irudayaraj].
In a separate set of experiments, we are investigating the aggregation of amyloid beta in the cerebro-spinal fluid collected from the transgenic Alzheimer mouse models. We are investigating if there are special factors at play in the CSF which makes the aggregation characteristics different from that of an artificial buffer solution. [Collaboration with V. Ravindranath].
In a separate set of experiments, we are investigating the aggregation of amyloid beta in the cerebro-spinal fluid collected from the transgenic Alzheimer mouse models. We are investigating if there are special factors at play in the CSF which makes the aggregation characteristics different from that of an artificial buffer solution. [Collaboration with V. Ravindranath].
