Designer Amyloids
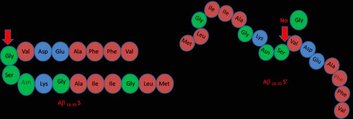
Focused perturbations of the critical structural features of the amyloid peptides can lead to a better understanding of the logic of amyloid assembly and to synthetic amyloids with novel properties. The small length of the peptide (40 or 42 residues) makes it amenable to solid phase peptide synthesis (we have put together a peptide synthesis and purification facility, see section 3B(c)). Amyloid beta 1-40 (Ab40) has a hydrophobic-polar-hydrophobic ‘tri-block’ sequence and adopts a hairpin-like core structure in the aggregated fibrillar state. It is easy to rationalize the hairpin structure: it allows interaction between the flanking hydrophobic blocks, while the turn region in the middle helps the burial of charged residues by facilitating a salt bridge formation. We use Ab18-35 as a model to investigate this structural logic of the core region of Ab40, and design variants which perturb these two dominant interactions. We design a single-glycine-deletion variant, which presumably makes the turn energetically costlier, and should therefore make this aggregation prone peptide more soluble. We also synthesize a block-permutated sequence, which introduces a cut in the turn region and bonds the two hydrophobic flanks resulting in a polar-hydrophobic-polar architecture. This is predicted to form stable soluble micelle-like structures. We measure their size, solubility and secondary structural properties using fluorescence correlation spectroscopy, solid state NMR, absorption spectroscopy, circular dichroism and Thio-T binding, and find both the predictions to be true. Our results support our understanding of the logic of amyloid assembly, and provide clues to designing potential bio-active designer amyloids.
